
In principle, in thermodynamics, for a process in a closed system, the quantity of heat transferred is defined by the amount of adiabatic work that would be needed to effect the change in the system that is occasioned by the heat transfer.

Adiabatic work is done without matter transfer and without heat transfer. For a process in a closed (no transfer of matter) thermodynamic system, the first law of thermodynamics relates changes in the internal energy (or other cardinal energy function, depending on the conditions of the transfer) of the system to those two modes of energy transfer, as work, and as heat. Thermodynamics has special concern with transfers of energy, from a body of matter, such as, for example a cylinder of steam, to the surroundings of the body, by mechanisms through which the body exerts macroscopic forces on its surroundings so as to lift a weight there such mechanisms are the ones that are said to mediate thermodynamic work.īesides transfer of energy as work, thermodynamics admits transfer of energy as heat. The total energy of a system is the sum of its internal energy, of its potential energy as a whole system in an external force field, such as gravity, and of its kinetic energy as a whole system in motion. Overview Conservation of energy Ī pre-supposed guiding principle of thermodynamics is the conservation of energy. The energy supplied by the fall of the weight passed into the water as heat. Work that does not change the volume of the water is said to be isochoric it is irreversible. Their motion scarcely affected the volume of the water. Mechanical work was done by the apparatus of falling weight, pulley, and paddles, which lay in the surroundings of the water. In this arrangement of apparatus, it never happens that the process runs in reverse, with the water driving the paddles so as to raise the weight, not even slightly. The modern day definitions of heat, work, temperature, and energy all have connection to this experiment. Joule estimated a mechanical equivalent of heat to be 819 ft Using these values, Joule was able to determine the mechanical equivalent of heat. Both the temperature change ∆T of the water and the height of the fall ∆h of the weight mg were recorded.
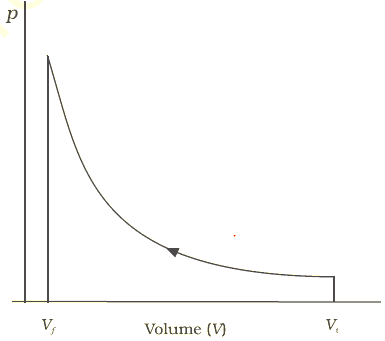
In this experiment, the motion of the paddle wheel, through agitation and friction, heated the body of water, so as to increase its temperature. In this paper, he reported his best-known experiment, in which the mechanical power released through the action of a "weight falling through a height" was used to turn a paddle-wheel in an insulated barrel of water. In 1845, the English physicist James Joule wrote a paper On the mechanical equivalent of heat for the British Association meeting in Cambridge. Joule's apparatus for measuring the mechanical equivalent of heat It has, as we know, as a measure, the product of the weight multiplied by the height to which it is raised. This effect can always be likened to the elevation of a weight to a certain height. We use here motive power to express the useful effect that a motor is capable of producing. "weight lifted through a height", was originally defined in 1824 by Sadi Carnot in his famous paper Reflections on the Motive Power of Fire, where he used the term motive power for work. The rate at which work is performed is power, measured in joules per second, and denoted with the unit watt (W). In the International System of Units (SI), work is measured in joules (symbol J). The surroundings also can perform work on a thermodynamic system, which is measured by an opposite sign convention.įor thermodynamic work, appropriately chosen externally measured quantities are exactly matched by values of or contributions to changes in macroscopic internal state variables of the system, which always occur in conjugate pairs, for example pressure and volume or magnetic flux density and magnetization.
This exchange results in externally measurable macroscopic forces on the system's surroundings, which can cause mechanical work, to lift a weight, for example, or cause changes in electromagnetic, or gravitational variables. Thermodynamic work is one of the principal processes by which a thermodynamic system can interact with its surroundings and exchange energy.


 0 kommentar(er)
0 kommentar(er)
